xef4 molecular shape|molecular shapes chart : Bacolod Now that we know the valence electrons of Xenon Tetrafluoride, it will be easier for you to draw its Lewis structure. This Lewis dot structure is a pictorial representation of valence . Tingnan ang higit pa Key Difference: DDR (DDR1), DDR2 and DDR3 are different types of SDRAM that are used in computers. DDR2 provides a faster transfer rate, bus clock and is more power-friendly compared to DDR1. DDR3 is an advanced version of the same technology. It enables faster bus speeds and higher peak throughput than earlier memory technologies.
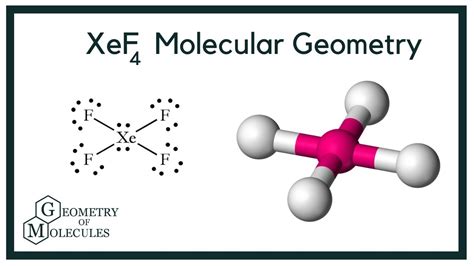
xef4 molecular shape,Although the bonds between Xenon and Fluorine atoms are polar, XeF4 is a nonpolar molecule. Wondering how? All the Xe-F bonds are in opposition with each other mutually, making the sum of dipole moment zero. As there are four electrons on the Xenon atom, which are localized as nonbonding . Tingnan ang higit paIn this molecule, we have one atom of Xenon and four atoms of Fluorine. We will calculate the valence electrons of both these atoms to determine the total number of valence electrons of XeF4. Valence electrons . Tingnan ang higit pa
xef4 molecular shape molecular shapes chartNow that we know the valence electrons of Xenon Tetrafluoride, it will be easier for you to draw its Lewis structure. This Lewis dot structure is a pictorial representation of valence . Tingnan ang higit paIt is easier to understand the molecular geometry of a given molecule once we know its Lewis structure. As Xenon has two lone pairs of electrons, it will take up a structure . Tingnan ang higit paThe central Xenon atom’s orbitals are hybridized, which results in the formation of new hybridized orbitals. Xenon has six electrons in its 5p orbitals and two electrons . Tingnan ang higit pa
Learn how to draw the Lewis structure, molecular geometry, and hybridization of XeF4, the chemical formula of xenon tetrafluoride. Find out the VSEPR theory and the octet rule that explain the square .
40K views 3 years ago. An explanation of the molecular geometry for the XeF4 (Xenon tetrafluroide) including a description of the XeF4 bond angles. The electron geometry for .
Xenon tetrafluoride is a chemical compound with chemical formula XeF 4. It was the first discovered binary compound of a noble gas. It is produced by the chemical reaction of xenon with fluorine: Xe + 2 F 2 → XeF 4This reaction is exothermic, releasing an energy of 251 kJ/mol. Xenon tetrafluoride is a chemical compound with chemical formula XeF4 XeF 4. It was the first discovered binary compound of a noble gas. It is produced by the . XeF4 lewis structure shape is a 3-D representation of how the atoms are arranged and what kind of geometry is suitable for them to maintain stability. The . Chemistry tutorial for the Lewis dot structure and molecular geometry of xenon tetrafluoride (XeF4).

What is the molecular geometry of XeF 4? The molecular shape of XeF 4 is square planar, or AX 4 E 2 using Valence Shell Electron Pair Repulsion (VSEPR) .Learn how to draw the Lewis structure, molecular geometry and electron geometry of XeF4 based on the VSEPR theory, steric number and sp hybridization. See examples and diagrams of XeF4 geometry and .XeF 4 contains 4 bonded and 2 nonbonded electron domains, giving an octahedral e - domain geometry and a square planar molecular geometry. (AX 4 E 2 ). A cartoon . Xenon tetrafluoride (XeF4) is a square planar, non-polar molecule. The Xenon atom has 4 bonding pairs of electrons and 2 lone (non-bonding) pairs of electro. Xenon tetrafluoride is a chemical compound with chemical formula XeF4 XeF 4. It was the first discovered binary compound of a noble gas. It is produced by the chemical reaction of xenon with fluorine, F2 F 2, according to the chemical equation: The infrared spectrum of XeF4 XeF 4 has absorptions at 161, 291, and 586 cm -1 (two bends, . XeF4; ClF4-Contributors and Attributions; This page explains how to work out the shapes of molecules and ions containing only single bonds. The examples on this page are all simple in the sense that they only contain two sorts of atoms joined by single bonds - for example, ammonia only contains a nitrogen atom joined to three hydrogen . Best Answer. Copy. The molecular shape of XeF4, or xenon tetrafluoride, is square planar. Its molecules are located in a square's corners around an atom in the center in a single place. Wiki User.The molecule of xenon tetrafluoride (with square planar shape XeF4 molecular geometry) is tilted at 90 degrees. It has a difference in electronegativity values between xenon and fluorine atoms, with fluorine’s pull the electron cloud being greater than xenon’s. As a result, it has no permanent dipole moment in its molecular structure.According to the VSEPR theory, the shape of the molecule is predicted by the total number of electron pairs (lone pairs + bond pairs) in the valence shell of the central Xe atom. Hence, there are 6 electron pairs. Since there are 4 fluorine atoms joined to xenon. So, there will be a 4bond pair of electrons.
xef4 molecular shape 1. The central atom, beryllium, contributes two valence electrons, and each hydrogen atom contributes one. The Lewis electron structure is. 2. There are two electron groups around the central atom. We see from Figure 9.2 that the arrangement that minimizes repulsions places the groups 180° apart. 3.
Xenon tetrafluoride is a chemical compound with chemical formula XeF 4.It was the first discovered binary compound of a noble gas. It is produced by the chemical reaction of xenon with fluorine:. Xe + 2 F 2 → XeF 4. This reaction is exothermic, releasing an energy of 251 kJ/mol.. Xenon tetrafluoride is a colorless crystalline solid that sublimes at 117 .
Six electron pairs around central atom four bond pairs, two lone pairs so square planar shape. $$ OSF^4: sp^3d $$ hybridized Five electron pairs around the central atom, five bond pairs so trigonal bipyramidal shape.
Around the central xenon atom there are # (8 + 4)# electrons, i.e. 6 electron pairs. The most energetically stable geometry around xenon is as an octahedron. And thus there are #4xx"Xe"-"F bonds"# in the plane, and 2 lone pairs of electrons, which are normal to the plane: AS with all these problems, it is important to recognize that the .
BrF5 Lewis Structure, Molecular Structure, Hybridization, Bond Angle and Shape. The chemical formula BrF5 represents Bromine Pentafluoride. It is an interhalogen compound and a strong fluorinating .XeF 4 contains 4 bonded and 2 nonbonded electron domains, giving an octahedral e - domain geometry and a square planar molecular geometry. (AX 4 E 2 ). A cartoon model of the electron density of the lone pairs of electrons, represented by translucent purple spheroids, can be toggled on and off. Note that the shapes of the spheroids do not . Conclusion. The Lewis structure for XeOF4. The molecular geometry of the XeOF4 molecule is square pyramidal. The hybridization state for the XeOF4 molecule is sp3d2. XeOF4 is a polar molecule. Happy learning!! Xenon Oxytetrafluoride is a colorless inorganic compound. Similar to other oxides of Xenon it is also very unstable and highly .Click the Symmetry Operations above to view them in 3D. XeF 4 belongs to the D 4h Point group and contains;. One C 4 rotation axis, one C 2 rotation axis (equivalent to C 4 2), Four C 2 axes perpendicular to the C 4 axis.. 4σ planes of symmetry,one σ h plane. One S 4 axis.. Some of the symmetry elements of a square-planar molecule such as XeF 4. .molecular shapes chart What is the molecular geometry of XeF 4?. The molecular shape of XeF 4 is square planar, or AX 4 E 2 using Valence Shell Electron Pair Repulsion (VSEPR) theory. Hence, the molecular geometry of XeF 4 has only 90 degree bond angles in the molecule. XeF 4 looks like this:

tetrahedral, octahedral, square planar, seesaw, or trigonal bipyramidal. Here’s the best way to solve it. Expert-verified. 100% (7 ratings) Share Share. Here’s how to approach this question. To solve this, first identify the number of bonding and non-bonding electron pairs around the central atom (Xe). Four fluorine atoms bond with these .1. The sulfur atom has six valence electrons and each fluorine has seven valence electrons, so the Lewis electron structure is. Four fluorenes are bonded to a central sulfur. Each fluorine has three lone pairs. Sulfur has one lone pair. With an expanded valence, this species is an exception to the octet rule.
Textbook Question. Values of Ea = 6.3 kJ>mol and A = 6.0 * 108>1M # s2 have been measured for the bimolecular reaction: NO1g2 + F21g2S NOF1g2 + F1g2 (b) The product of the reaction is nitrosyl fluoride. Its formula is usually .
xef4 molecular shape|molecular shapes chart
PH0 · xef4 valence electron
PH1 · vsepr shapes chart
PH2 · naming molecular compounds quizlet
PH3 · naming molecular compounds pdf
PH4 · naming molecular compounds
PH5 · molecular shapes chart
PH6 · ionic vs molecular compounds examples
PH7 · if4 shape and angle
PH8 · Iba pa